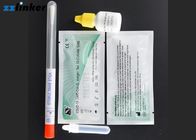
Covid-19 (SARS-CoV-2) Virus Rapid Antigen Test Kit CE FDA Approved
Limitations of inspection methods:
1. This test kit is only used for in vitro diagnosis.
2. This test kit is only used to detect human throat or nasal swab extracts. The results of other specimens may be wrong.
3. This test kit is only used for qualitative detection and cannot indicate the level of SARS-CoV-2 antigen in the specimen.
4. This test kit is only a clinical auxiliary diagnostic tool. If the result is positive, it is recommended to use other methods for further examination in time and the doctor’s diagnosis shall prevail.
Materials provided:
1) Sterilized Swab
2) Antigen extraction tube
3) Extraction Reagent
4) Test device
Materials required but not provided:Timer.
[Storage conditions & period of validity]
1. Store at 4℃~30℃, and it is valid for 24 months.
2. After the aluminum foil bag is unsealed, the test device should be used as
soon as possible within one hour.
Storage and Expiry:
| Room Tempreture |
4-30 degree |
| Weather Request |
Dry and no light, no freeze |
| Expiry Date |
24 months |
Specimen Collection:
1. Pharyngeal swab Sample:
Let the patient's head tilt slightly, mouth open, and make "ah" sounds, exposing the pharyngeal tonsils on both sides. Hold the swab and wipe the pharyngeal tonsils on both sides of the patient with a little hard back and forth at least 3 times.
2. Nasal swab Sample:
Let the patient's head relax naturally, and slowly rotate the swab against the wall of the nostril into the patient's nostril to the nasal palate, and then slowly remove it while wiping. Using the same swab, wipe the other nostril in the same way;



Sample Transport and Storage:
After Swab specimens were collected, swab can be stored in extraction reagent provided with the kit. Also can be stored by immersing the swab
head in a tube containing 2 to 3 mL of virus preservation solution (or isotonic saline solution, tissue culture solution, or phosphate buffer).
Freshly collected specimens should be processed as soon as possible, but no later than one hour after specimen collection. Specimen collected may be stored at 2-8℃for no more than 24 hours; Store at -70 ℃for a long time, but avoid repeated freeze-thaw cycles.
Specimen Preparation:
1. Take out the extraction tube, add 8 drops (about 0.3 mL) of the extraction reagent into the extraction tube, and put it on the workbench.
2. Put the swab specimen into the extraction tube, rotate the swab for about 10 seconds, and press the swab head against the tube wall to release the antigen in the swab.
3. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab. so as to remove as much liquid as possible from the swab. Dispose of swabs according to biohazard waste disposal method.
4. Insert a dropper tip into the extraction tube tightly.
Test Procedure:
Read the instructions carefully before use and bring test device, extraction reagent and specimens were restored to room temperature.
1. Open the package and take out the test device.
2. Insert a dropper tip into the extraction tube tightly, put two drops into the specimen well (s) of the test device, and start the timer.
3. Interpret the results within 20 minutes. Strong positive results can be reported within 20 minutes, however, negative results must be reported after 20 minutes, and the results after 30 minutes are no longer valid.
Interpretation of test results:
Negative result: if there is only a quality control line C, the detection line T is colorless, indicating that SARS-CoV-2 antigen has not been detected and the result is negative.
Positive result: if both the quality control line C and the detection line T appear, indicating that SARS-CoV-2 antigen has been detected and the result is positive.
Invalid result: if the quality control line C is not observed, it will be invalid regardless of whether there is detection line T (as shown in the figure below), and the test shall be conducted again
More Contact Information:
Echo Chai (Miss) ---Export Department
Zhengzhou Linker Medical Equipment Co., Ltd.
Web: www.zzlinker.com
E-mail/Skype: sale@zzlinker.com
Tel: +86 371 63937550
Whatsapp/Wechat: +86 18860361012
 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!